|
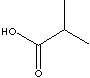
ISOBUTYRIC ACID |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
PRODUCT IDENTIFICATION |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS NO. | 79-31-2 |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EINECS NO. |
201-195-7 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FORMULA | (CH3)2CHCOOH | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MOL WT. |
88.11 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| H.S. CODE | 2915.60 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
TOXICITY |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SYNONYMS | 2-Methylpropionic acid; 2-Methylpropanoate; Isobutric acid; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2-methylpropanoic acid; Dimethylacetic acid; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
SMILES |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
CLASSIFICATION |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PHYSICAL STATE |
clear liquid |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MELTING POINT | -45 C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BOILING POINT |
153 - 155 C |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SPECIFIC GRAVITY | 0.948 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SOLUBILITY IN WATER | miscible | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| pH |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VAPOR DENSITY | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
AUTOIGNITION |
425 C |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
REFRACTIVE INDEX |
1.393 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
NFPA RATINGS |
Health: 3 Flammability: 2 Reactivity: 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FLASH POINT |
55 C |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| STABILITY | Stable under ordinary conditions | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
DESCRIPTION AND APPLICATIONS |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| n-Butyric acid is a saturated four-carbon carboxylic acid which is a clear liquid, with a suffocating odor and with melting point -6 C and boiling point 164 C. It occurs naturally in rancid butter, in much animal fat and in plant oil. It is soluble in water, ethanol and ether. It can be obtained by the fermentation of sugar or starch, brought about by the addition calcium carbonate or by the oxidation of butanol in the presence of potassium permanganate. There is a structural formula called isobutyric acid which can not be obtained by the fermentation but by the oxidation of isopropyl alcohol with potassium bichromate and sulfuric acid. Isobutyric acid melts at -47 C and boils at 154 C. It is slightly soluble in water but soluble in ethanol, ether and organic solvents. Butyric acid is a strong acid and reacts with bases, strong oxidants and metals. It is used to eliminate calcium in leather industry. Butyric acid family products, such as their esters, are used for the production of plastics, plasticizers, surfactants and textile auxiliaries. They are used in food additives, flavorings, varnishes, perfumes, pharmaceuticals and disinfectants. Aminobutyric acid is a four-carbon carboxylic acid, to which an amino group is attached. There are three structural isomers, alpha, beta, gamma-. Alpha-aminobutyric acid is has an amino group substituted at the alpha, or 1 position on the carbon atom next to the acid group, while gamma-aminobutyric acid (GABA) at the terinal carbon (the gamma, or 4 position). Natural GABA exits in L-form only and can be found in plant and animal tissues. It is formed in the metabolism of L-glutamic acid. It is the central nervous system postsynaptic inhibitory transmitter in the brain but is also found in several extraneural tissues, including kidney and pancreatic islet beta cells. Gamma- hydroxy butyric acid is an intermediate occurring in metabolism of GABA. There are several hydroxy butyric acid occur at elevated levels in some metabolic disorders. In biological systems The GABA family products are the parent compounds for a number of psychoactive drugs covering sedation/hypnosis, anxiolysis, anticonvulsant activity, muscle relaxation and anterograde amnesia. Some examples are benzodiazepines, baclofen, bicuculline, barbiturates, picrotoxin, neurosteroids and the general anesthetics. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SALES SPECIFICATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
APPEARANCE |
clear liquid |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
PURITY (GC) |
99.0% min |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
ISOMER IMPURITY |
0.5% max |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
WATER |
0.2% max |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
COLOR, APHA |
15 max |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TRANSPORTATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PACKING | 190kgs in drum | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HAZARD CLASS | 8 (Packing group: III) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UN NO. | 2529 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GENERAL DESCRIPTION OF CARBOXYLIC ACID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Carboxylic acid
is an organic compound whose molecules contain carboxyl group and have the
condensed chemical formula R-C(=O)-OH in which a carbon atom is bonded to an
oxygen atom by a solid bond and to a hydroxyl group by a single bond), where R
is a hydrogen atom, an alkyl group, or an aryl group. Carboxylic acids can be
synthesized if aldehyde is oxidized. Aldehyde can be obtained by oxidation of
primary alcohol. Accordingly, carboxylic acid can be obtained by complete
oxidation of primary alcohol. A variety of Carboxylic acids are abundant in
nature and many carboxylic acids have their own trivial names. Examples are
shown in table. In substitutive nomenclature, their names are formed by adding
-oic acid' as the suffix to the name of the parent compound. The first character
of carboxylic acid is acidity due to dissociation into H+ cations and
RCOO- anions in aqueous
solution. The two oxygen atoms are electronegatively charged and the hydrogen of
a carboxyl group can be easily removed. The presence of electronegative groups
next to the carboxylic group increases the acidity. For example, trichloroacetic
acid is a stronger acid than acetic acid. Carboxylic acid is useful as a parent
material to prepare many chemical derivatives due to the weak acidity of the
hydroxyl hydrogen or due to the difference in electronegativity between carbon
and oxygen. The easy dissociation of the hydroxyl oxygen-hydrogen provide
reactions to form an ester with an alcohol and to form a water-soluble salt with
an alkali. Almost infinite esters are formed through condensation reaction
called esterification between carboxylic acid and alcohol, which produces water.
The second reaction theory is the addition of electrons to the
electron-deficient carbon atom of the carboxyl group. One more theory is
decarboxylation (removal of carbon dioxide form carboxyl group). Carboxylic
acids are used to synthesize acyl halides and acid anhydrides which are
generally not target compounds. They are used as intermediates for the synthesis
esters and amides, important derivatives from carboxylic acid in biochemistry as
well as in industrial fields. There are almost infinite esters obtained from
carboxylic acids. Esters are formed by removal of water from an acid and an
alcohol. Carboxylic acid esters are used as in a variety of direct and indirect
applications. Lower chain esters are used as flavouring base materials,
plasticizers, solvent carriers and coupling agents. Higher chain compounds are
used as components in metalworking fluids, surfactants, lubricants, detergents,
oiling agents, emulsifiers, wetting agents textile treatments and emollients,
They are also used as intermediates for the manufacture of a variety of target
compounds. The almost infinite esters provide a wide range of viscosity,
specific gravity, vapor pressure, boiling point, and other physical and chemical
properties for the proper application selections. Amides are formed
from the reaction of a carboxylic acids with an amine. Carboxylic acid's
reaction to link amino acids is wide in nature to form proteins (amide), the
principal constituents of the protoplasm of all cells. Polyamide is a polymer
containing repeated amide groups such as various kinds of nylon and
polyacrylamides. Carboxylic acid are in our lives.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||